Regulatory
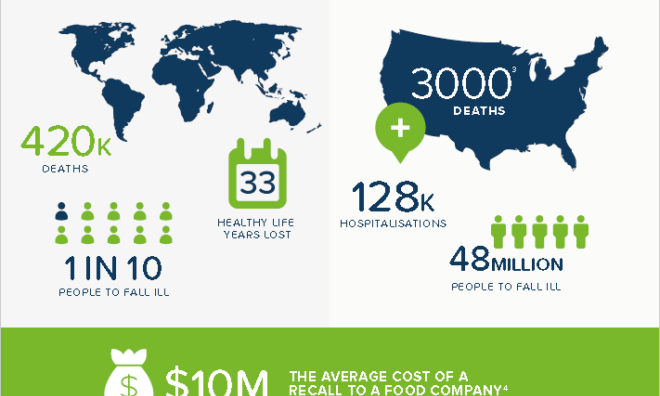
Drug Companies Cited Over Lack of Stability Testing
The US FDA provides a comprehensive public record of the citations and warning letters [1] issued to Pharmaceutical Companies. This provides an excellent resource for those looking for guidance to improve quality and mitigate risk.
8th October 2020