Press releases
Autoscribe Releases LIMS System Validation White Paper
10th September 2020
Autoscribe Informatics, a leading global laboratory informatics provider, announced the release of a new white paper on the validation of Laboratory Information Management Systems (LIMS). The white paper discusses what LIMS validation is and the steps involved, the responsibilities of the customer and supplier, how GAMP5 affects LIMS validation, and the deliverables you can expect from a LIMS validation project.
LIMS solutions are widely used by companies subject to regulation by the FDA and equivalent international bodies. To be suitable for use in regulated industries the LIMS must be shown to fulfil its intended purpose and work in the way it is designed to work. While it is the responsibility of companies themselves to prove the system has been validated to the required level, Autoscribe has over 30 years’ experience in building and implementing LIMS for regulated organizations.
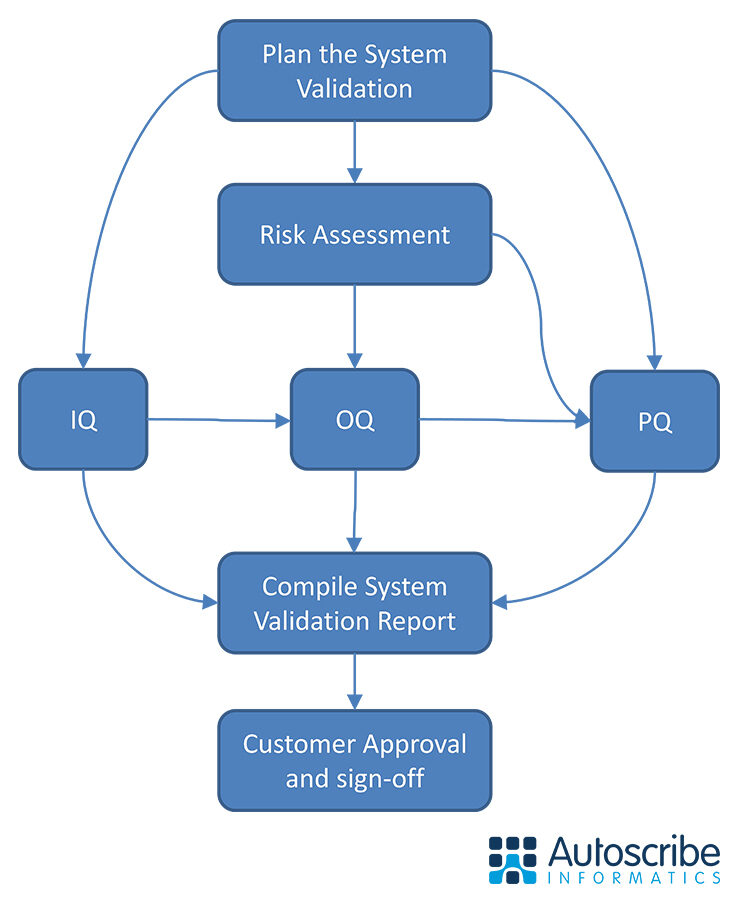
The white paper distils that knowledge into an easy to follow reference that can serve as a foundation for LIMS administrators and regulatory compliance managers on how to approach LIMS validation. It can help them understand the steps involved including creation of a user requirement specification, the development of a functional specification, the various stages of qualification testing, and production of a final summary report.
“As well as adhering to the many standards, such as FDA 21 part 11, ISO/IEC 17025, good laboratory practice (GLP) and good automated manufacturing practice (GAMP), regulated companies must satisfy themselves that their information management systems perform as expected and are fit for purpose,” said Simon Wood, Product Manager at Autoscribe Informatics. “This system validation white paper gives LIMS practitioners a tried and trusted framework to work form to help ensure the system has been implemented and tested with the necessary rigor to be sure this is so.”
LIMS System Validation White Paper
Find out about LIMS Validation