Blog: Business
Can One LIMS Cover All Your Needs?
LIMS can cover many different requirements in your business. But can one LIMS cover all of them?
12th November 2021

Are you constantly surprised by the variety of work your laboratory is asked to do? While the main purpose of your lab may be to test the quality of manufactured products, are you presented with air filters to test for microbiological contamination, or packages of pills for stability testing? In your lab testing the safety of water for domestic consumption are you asked to check the quality of a new reagent or consumable? In your food testing lab do people turn up with a sample of product returned by a consumer that has to be analyzed immediately as part of a CAPA process? If this is the case it is vital to have a LIMS that can handle the full spectrum of testing that you may be asked to perform. A system optimized for one function but lacking the flexibility to manage others will negatively impact the lab and the perception of the lab.
LIMS are used in very different ways depending on the work being performed, the various types of samples to be analyzed, and the information to be reported. It is worth exploring some of the ways in which LIMS is used and what needs to be tracked in each case.
LIMS For Manufacturing Processes
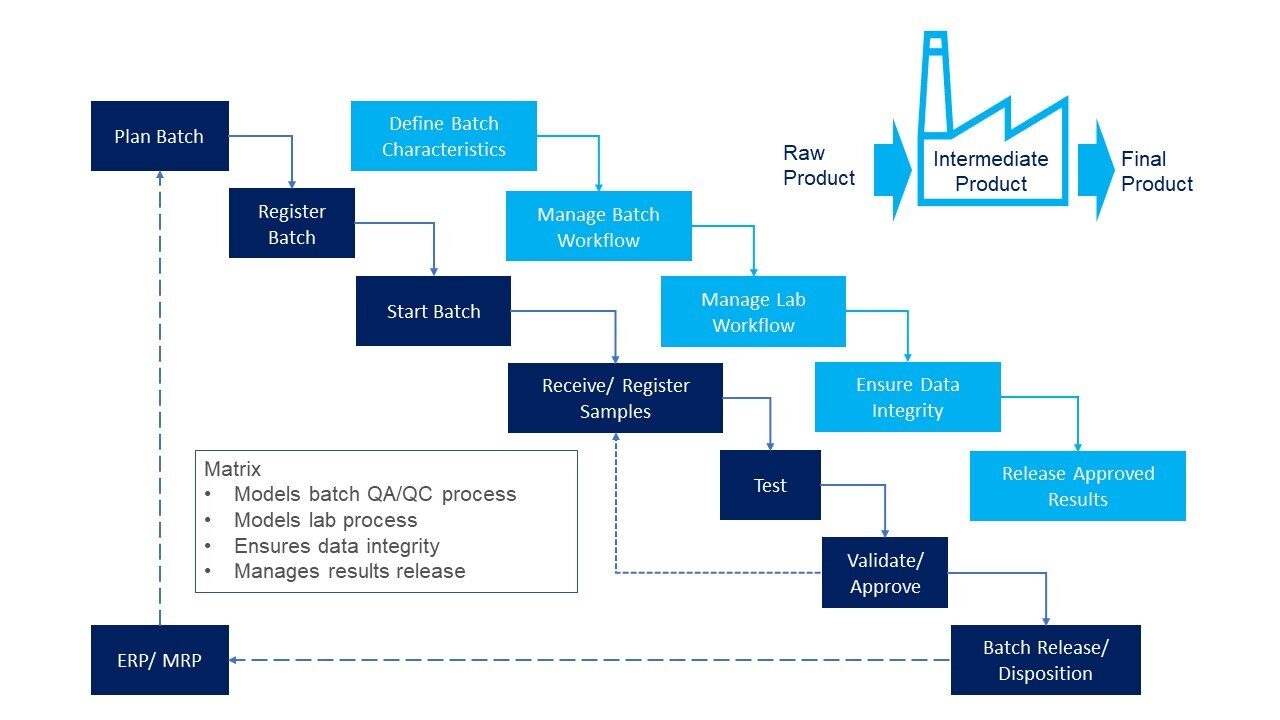
LIMS used in manufacturing need to track batches of product, and the testing performed at each stage of the manufacturing flow. A recipe management module within the LIMS links raw ingredients and intermediate products to a final product. Therefore, for each batch of product the raw materials and intermediates used can be tracked, together with the samples taken for analysis from various points in the production of each of the batches. This provides a batch genealogy which, should a problem be identified, allows complete traceability, and helps identify the root cause of the problem, and potentially any other batches that could be affected. Because known products are being tested it is also simple to define the exact set of tests that are required.
Fundamentally little changes between manufacturing lines for pharmaceutical and medical devices, and manufacturing lines for paint or screws. The main difference is that regulated industries (often those which can affect human health) require tighter control of the quality assurance, for instance validating the LIMS before use and validating any changes in software or configuration before they are used in anger.
LIMS for Ad Hoc Testing
Rather than link samples back to a batch, samples may be taken as a result of isolated or random events. The LIMS must be able to cope with these ad hoc samples, which may or may not be linked to batches or lot numbers. Ad hoc samples may, for example, be required because of a potential issue with a returned product from a consumer, or random sampling of reagents for quality control purposes. Samples are tested and results passed back to the QA team who will take appropriate action depending on the results. This type of testing is very similar to that carried out by a contract testing Laboratory, which is probably the type of laboratory most widely supported by a LIMS.
As well as Ad-hoc, or discrete, testing being used in QC laboratories within manufacturing when additional testing is required, this type of testing is also widely used in general contract and QC laboratories. Contract laboratories, for instance, will typically be testing discrete samples for specific analytical tests. You still might link the testing back to a lot or batch number, and/or location in water/environmental testing, but there is no recipe management linking multiple samples from every stage in a production line. Because of the wide variety of industry types in which it is used ad-hoc testing is probably the most common form of testing managed by a LIMS.
Sterility/HACCP Testing
Sterility testing is used to prove cleanliness of manufacturing environments. It is commonly used in the food and beverage, pharmaceutical and medical device industries, among others. In the food industry it is a key part of any HACCP program (Hazard Analysis and Critical Control Point).
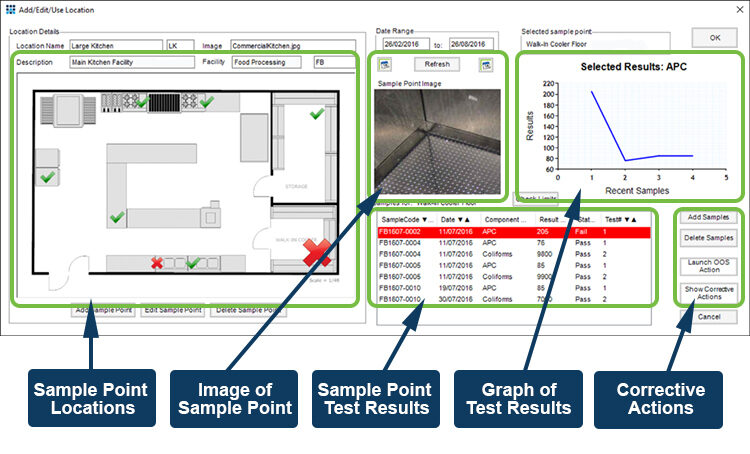
For sterility testing specific locations are tested regularly to detect the presence or build-up of potentially dangerous biological and chemical hazards that might affect the production process. Sampling points often include air filters, work surfaces and touch points such as handles. The key to this type of testing is linking the sample back to the location from which it was taken, and to be alerted to significant changes that may take place over time. The Xybion LIMS environmental module does exactly this; managing the samples, sampling schedule, and sampling points, and linking this information to provide a graphical representation of the state of the monitored facilities.
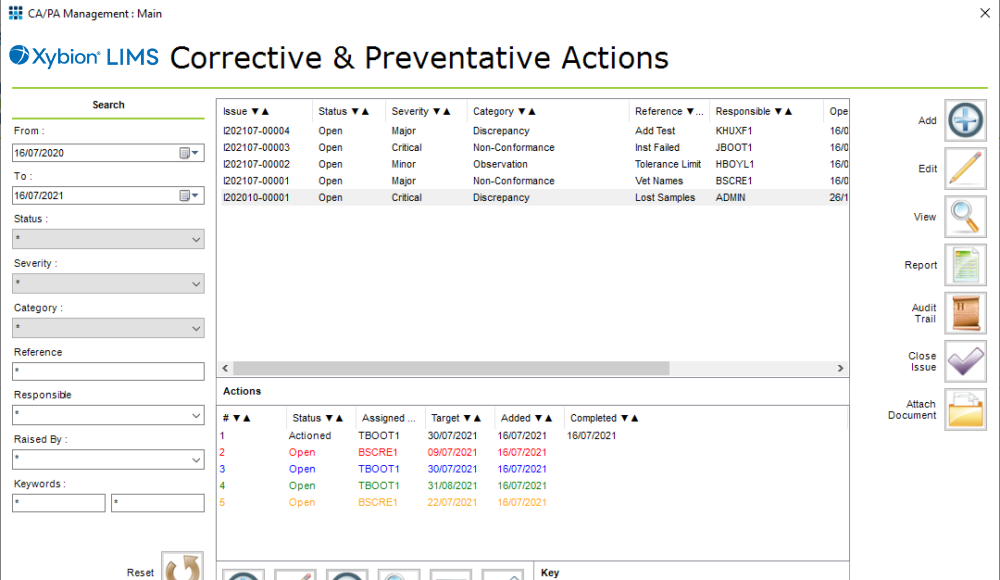
If issues are identified then a CAPA cycle can be started to investigate and resolve the problem before production needs to be stopped, or worse, batches of product need to be recalled from the supply chain. Xybion LIMS includes CAPA management functionality that integrates, in a single system, the identification of possible issues with the actions require to resolve them.
Stability Study Testing
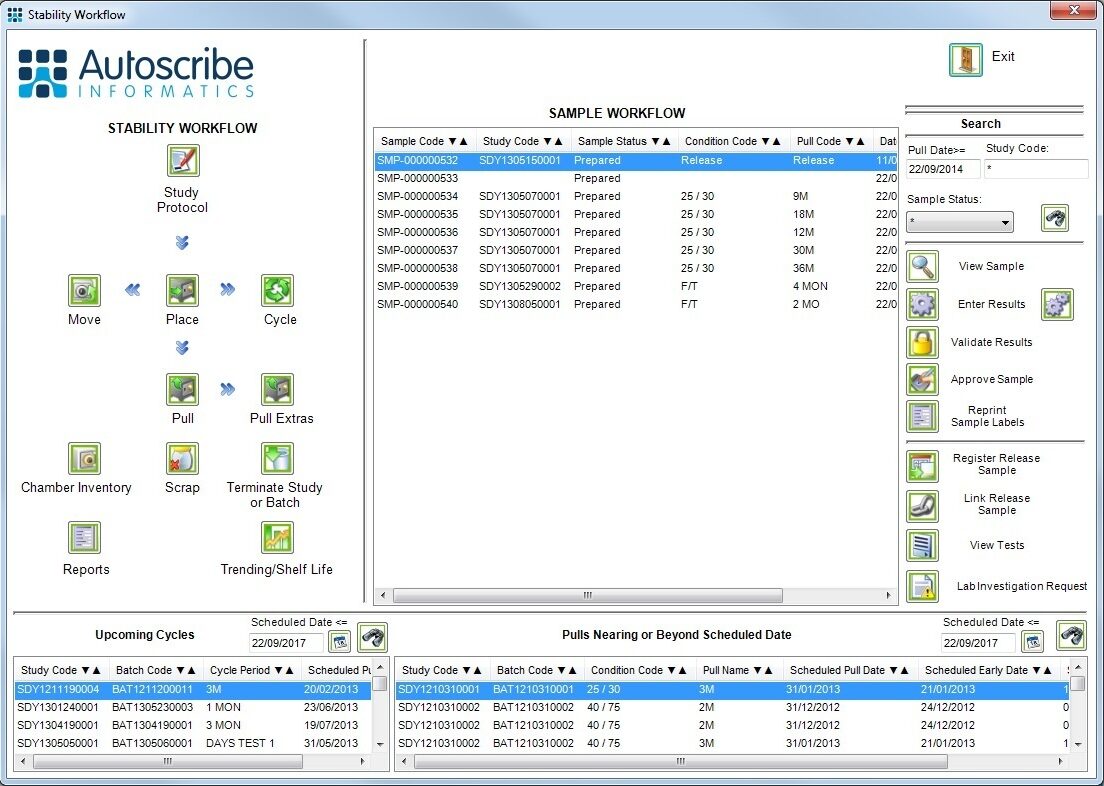
Stability testing is used to test and predict the shelf life of products. Any product that has a shelf life will potentially need stability testing; pharmaceuticals medical-devices, and food and beverage being the key areas. In this scenario the LIMS must manage the stability study protocols, which include how long and under what conditions samples must be stored, and the samples to be tested over time.
Samples are pulled from storage at the appropriate time (typically 1, 3, 6, 12 months etc. in the pharmaceutical world) and tested. The test results graphically plotted for each batch over time provide the required data to project the shelf life of the product
LIMS Configuration is Key
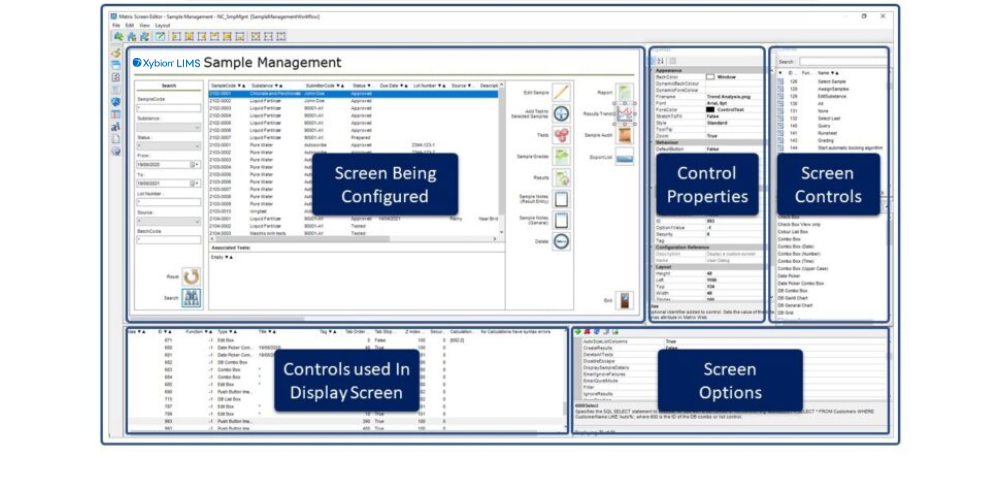
These are just four use scenarios, and many more will exist, that show the wide variety of ways a LIMS is used. For many software providers providing a single solution to all these scenarios is challenging. Over its last 32 years of operation Autoscribe Informatics has always provided highly flexible LIMS solutions that meet the needs of multiple types of organization and address multiple markets with a single solution. Its configuration editor, called the Matrix Configuration tools, can adapt the LIMS to multiple scenarios. The underlying software is not changed during configuration and remains separate from the configuration of the user interface. This allows users to upgrade their systems without affecting the system configuration which includes workflow and screen designs.
Matrix Configuration tools are a Low-Code Development Platform (LCDP), allowing configuration without software coding. This means that appropriately trained staff at a customer site, not normally the IT team, can undertake configuration tasks, therefore removing a potential bottleneck due to limited IT resources.
The benefits of Matrix Configuration tools include:
- Ability to configure the system to precisely meet user and business requirements
- Future proofing your investment through the ability to rapidly meet changing requirements and business processes
- Autoscribe support for all user developed configurations
- Enable seamless upgrade to newer versions without re-configuration
Supporting Multiple Uses
The key here is the ability to configure the LIMS to meet any user and business requirement using exactly the same software. This allows us to support configurations that enable all the above scenarios simultaneously. Need to link samples to batches in a manufacturing flow? No problem. Need to perform ad-hoc testing? No problem. Need to carry out sterility checks in your manufacturing environment? No problem. Need to derive shelf-life statistics for product? No problem. Need to take corrective action when a problem surfaces using the CAPA approach? No problem. Indeed, we can easily combine all these scenarios and provide an organization with a single solution that meets all their testing needs, all without writing a single line of code.
Industries and market areas can have specific requirements. For instance. A LIMS for the water industry and LIMS optimized for Veterinary Laboratories will have different specific requirements. The Matrix configuration tools allow Autoscribe to provide pre-configured starter solutions for many of these key market areas. These provide the starting point for new system deployments and allow solutions to go-live quickly. Even these though can be combined and re-configured to create the unique solution you require for your specific laboratory.
With Xybion LIMS you get a LIMS that can cover all your laboratory informatics needs.