
Radiopharmacy LIMS
Prepare and manage radioactive preparations for patient use with absolute confidence.
Matrix Gemini LIMS
The complete radiopharmacy solution
Matrix Gemini LIMS for radiopharmacy has been developed in collaboration with leading radiopharmacists. It helps manage the production of specialized radioactive preparations that must be closely controlled and monitored throughout their life. It is a fully pre-configured out-of-the-box solution to meet the exacting requirements of individual radiopharmacy and nuclear medicine departments.
The Matrix Gemini LIMS for Radiopharmacy Information Management System is a fully adaptable solution to meet the requirements of individual radiopharmacies. It is suitable for radio pharmacies that operate as stand-alone units and those that provide radioactive products to other hospital units. Its flexibility also means that it can support the provision of radioactive material as single injections or as a bulk supply of multiple injections for separation later into individual doses for patient scans. The system can produce the shipping documents required to allow the material to be delivered to the point of use either within or external to the hospital where the products are created.

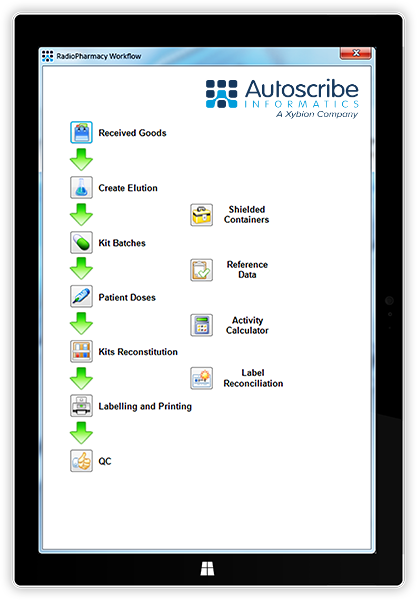
Radiopharmacy/Nuclear Medicine
Specific features:
- A cost-effective, configurable, information management system suitable for all radiopharmacy facilities
- Provides full traceability from incoming orders through to production and shipping for regulatory compliance
- Inventory management including expiration dates - expired materials are automatically flagged and made unavailable for the production process
- Stores dose calibrator check records, generator elution logs and QC test results for later auditing
- Ensures workflows, procedures and checklists followed and recorded
- Outputs all required labels with or without barcodes
- Records internal and external facility checks and deviations
- Manages materials in the "holding store" for safe disposal
- Can provide audit reports to confirm regulatory guidelines have been met
- Can operate as a paperless system to minimize contamination and microbial infection
PDF DOWNLOAD
Radiopharmacy management system
For more detailed information, see our brochure.


Related content
![Radiological]()
Case studies
Matrix Gemini LIMS Meets Radiological Testing Challenges
Discover how one Radiological Laboratory uses Matrix Gemini LIMS to manage complex radiochemistry data.
Solution: LIMS
US Radiological Laboratory
![Bee naturalles IRM9qg Zdl W0 unsplash]()
White papers
How To Buy A LIMS The Definitive Guide
This LIMS Selection Guide aims to help anyone considering the purchase of a new or replacement Laboratory Management System (LIMS).
![Cavendish Nuclear logo]()
Case studies
UKAS Accredited Lab Manages its Own Configurations of Matrix Gemini LIMS
Cavendish Nuclear use the flexible Matrix Gemini LIMS configuration tools to make their own changes as requirements change.
Solution: LIMS
Cavendish Nuclear
Let’s Talk
Ready to get started? Contact us today.
Let’s connect and we’ll arrange a Matrix Gemini LIMS demo.



